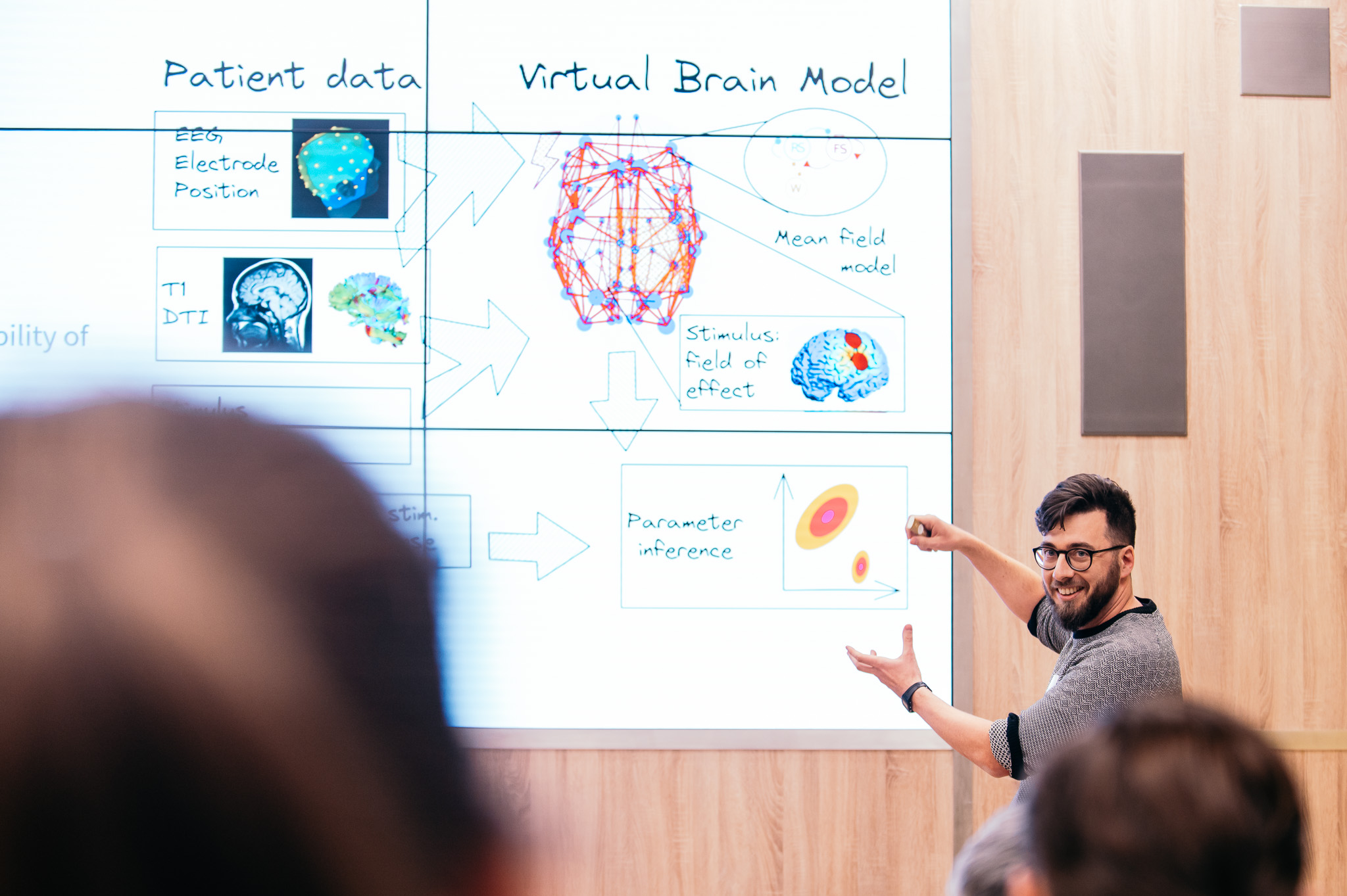
Design & development details of RT-PCR tests published

At the start of the pandemic, our team moved from our primary field, oncology research, to R&D of RT-PCR tests with one main goal - to help. In MultiplexDX International, we have been clear about this from the beginning. That is also why we have recently made the whole design, including components of our RT-PCR tests, available to the whole scientific community.
The article, in which we disclosure the design and components of seven RT-PCR tests, was recently published in the prestigious scientific journal Microbial Biotechnology. This publication follows another previously published paper about our COVID-19 tests in the Scientific Reports.
In addition, our tests have been evaluated in several certified laboratories (e.g. AnalytX, The Laboratory for RNA Molecular Biology at The Rockefeller University, the RIVM National Institute for Public Health and the Environment in the Netherlands, etc.). In addition, they have passed the External Quality Assessment, organized by the European Centre for Disease Prevention and Control (ECDC), which has been attended several times and with several of our tests by our scientific partner Biomedical Research Center of the Slovak Academy of Sciences.
Our tests also have an IVD certificate from the State Institute for Drug Control Slovakia, which is clear evidence that they have passed proper clinical validation with all the necessary and required parameters of the regulatory authority. At the same time, these IVD-certified tests are in the European database and have undergone further external evaluation
And last but not least, our company holds the important ISO 13485:2016 certificate, which is proof that we meet the highest standard for quality development and production of diagnostic tests.
We at MultiplexDX hope this might help also other scientific teams to speed up their R&D for any future COVID-19 RT-PCR tests. As we are mainly a scientific team, we envisage sharing of know-how as an accelerator of scientific research.
About MultiplexDX
MultiplexDX is one of the most innovative biotech corporations, created to bring its revolutionary technologies to the market of personalized molecular diagnostics. The company has representation in both U.S. and European markets. The collaborators of MultiplexDX are from the world’s most prestigious scientific organizations including the National Cancer Institute, Rockefeller University, Albert Einstein University, Vanderbilt University, Cornell University, Queens University (Canada), Hebrew University of Jerusalem (Israel), and the Max Delbrück Center for Molecular Medicine (Germany).
MultiplexDX IP-based and innovative platform merges histopathology methods, biomarker quantification, visualization and gene expression with a single-cell resolution by combining MDX proprietary visual and sequencing technologies into one diagnostic test. This cross-validation approach eliminates diagnostic errors and creates 100% precise cancer profiling for each patient which allows clinicians to suggest specific, personalized cancer treatment.
Find out more about MultiplexDX on Corporate website, Facebook, LinkedIn, Twitter